2020 Volume 45 Issue 3 Pages 117-129
2020 Volume 45 Issue 3 Pages 117-129
Silica nanoparticles (SiO2 NPs) are widely used in daily life and can enter organisms through several pathways, often causing unpredictable toxicity. Although SiO2 NPs are known to cause damage to the respiratory system, little is known about their oral toxicity, and their potential harm to the reproductive system is unclear. In this study, we used a Caenorhabditis elegans model to clarify SiO2 NPs oral toxicity in vivo and explore their effect on the reproductive system. We exposed C. elegans to 0.25, 0.5 and 1 mg /mL SiO2 NPs for 24 hr. Our results showed that SiO2 NPs exposure for 24 hr did not affect nematode survival rates, but did affect, to varying degrees, the reproduction, development, and movement of nematodes, with nematode fecundity being the most sensitive to SiO2 NPs toxicity. The NPs exposed group showed enhanced germ cell apoptosis and increased oxidative stress as seen through an increase in ROS and malondialdehyde (MDA), and decrease in reduced glutathione (GSH). N-acetyl-L-cysteine (NAC), an antioxidant, negated SiO2 NPs effect on germ cells and restored nematodes reproductive ability. We also found that SiO2 NPs could affect the expression of genes related to metal detoxification, oxidative stress, and apoptosis. The expression of metallothionein coding genes mtl-1 and mtl-2 changed most significantly among the tested genes. We demonstrated that SiO2 NPs could enhance germ cell apoptosis by inducing oxidative stress, providing a new area for studies of the mechanism of SiO2 NP toxicity.
Silica nanoparticles (SiO2 NPs) are an important nanotechnology product. They are widely used in electronics, biosensors, drug delivery and surface science because they are easy to prepare, have low polymerization rates, and surface modification is a simple process (Rong et al., 2017). Such widespread use of nanoparticles has raised concerns about potential effects on health from environmental exposure. Previous studies have shown that SiO2 NPs cause pulmonary toxicity, cardiovascular toxicity and immunotoxicity in vivo (Yang et al., 2017; Feng et al., 2018; Kim et al., 2014). However, in these studies, the exposure route studied was mainly inhalation exposure, and oral exposure was rare. Due to the extensive use of SiO2 NPs in food processing, production and storage, the average person consumes 1.8 mg/kg of nano-sized silica every day from food (van der Zande et al., 2014). Moreover, the use of silica nanoparticles as a drug carrier is increasing in clinical practice, so it is necessary to investigate the toxicity of nano-silica exposure from oral administration in vivo. A previous study observed tissue damage in the testes of mice fed silica nanoparticles (Hassankhani et al., 2015). In addition, studies have shown that inhalation exposure to SiO2 NPs have adverse effects on the reproductive system and can cause pathological changes in testicular tissue in male mice and reduce the fecundity of pregnant mice (Pinto et al., 2018; Ren et al., 2016; Yamashita et al., 2011). There is, however, still a significant gap in our understanding of the underlying mechanism of SiO2 NPs action on the reproductive system.
Nowadays, in vivo toxicity experiments are not limited to mice and rats and Caenorhabditis elegans is being more often used as a model organism to evaluate the safety of exogenous compounds, including nanomaterials. As a model organism, C. elegans has many advantages, such as a short life cycle, ease of cultivation, high reproductive capacity, cellular simplicity, and availability of the whole genome sequence (Kaletta and Hengartner, 2006). C. elegans lack a respiratory system and have a hard outer stratum corneum, which limits their use in investigating effects on inhalation and skin exposure pathways. However, nematodes can continuously ingest liquids and suspended environmental particles through the throat pump mechanism, which makes them a potential oral exposure model (Avery and You, 2012). Furthermore, C. elegans is very sensitive to external environmental changes and many of its basic physiological processes and stress responses are conserved in higher eukaryotes, including humans (Swain et al., 2004). Studies have found that the nanoparticle toxicity in nematodes is similar to that seen in mammals (Kim et al., 2019; Ratnasekhar et al., 2015). Because of the high cost and long experimental timeframes incurred using mouse and other mammalian systems, nematodes have become a more popular model for evaluating nanoparticle toxicity. In this study, we exposed nematodes to SiO2 NPs and studied the effects on survival, reproduction, development, and movement. We also examined indicators of germ cell apoptosis, oxidative stress, and gene expression. The overall goal of this study was to develop the foundation for oral safety assessment of nano-silica and to provide new research paths for uncovering the mechanism of reproductive toxicity of nano-silica.
SiO2 NPs were made by Shanghai Keyan Industrial Co. Ltd. (Shanghai, China). Reduced glutathione (GSH) and malondialdehyde (MDA) assay kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Fluorescein isothiocyanate (FITC), acridine orange (AO), N-acetyl-L-cysteine (NAC) and 2′,7′-dichlorofluorescein diacetate (DCFDA) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
30 nm diameter SiO2 NPs without coating were used in the experiments. Nanoparticle purity was > 99.5%. The shape and size of SiO2 NPs were examined by transmission electron microscopy (TEM, TECHNAI G2, FEI, Eindhoven, Netherlands). Nanoparticle suspensions (0.25, 0.5 and 1 mg/mL) were prepared in K-medium (32 mM KCl and 51 mM NaCl) (Brenner, 1974), a liquid medium used for C. elegans maintenance. These concentrations were selected to avoid nanoparticle aggregation (Fig. S1). The hydrodynamic size and zeta potential of SiO2 NPs in K-medium were assessed by the Malvern Zetasizer Nano S90 (Malvern Instruments Ltd., Malvern, UK). To detect the change of the nanoparticles hydrodynamic size, we tested the hydrodynamic size of nanoparticles at 0, 1, 2, 4, 8, 12, and 24 hr, respectively.
Worm culture and treatmentThe nematodes used in this study were all wild-type N2 hermaphrodite, originally derived from the Caenorhabditis Genetics Center (CGC; Minneapolis, MN, USA). Worms were cultured on nematode growth medium (NGM) plates inoculated with E. coli OP50 at 20°C (Brenner, 1974). For age-synchronized testing, we used the standard hypochlorite procedure to elute and lyse the gravid hermaphrodites to obtain eggs. Eggs were incubated overnight in liquid media without E. coli OP50 to obtain age-synchronized nematodes. Experiments were carried out using L4-stage larvae (age-synchronized worms grown on food-containing plates for 48 hr). Nematodes were exposed food containing different concentrations of SiO2 NPs, with the control group exposed to K solution containing E. coli OP50.
Fluorescein isothiocyanate (FITC) labeled nanoparticlesTo determine whether the worms could ingest SiO2 NPs, FITC modified with 3-aminopropyltrimethoxysilane (FITC-APTMS) was used to label SiO2 NPs. APTMS chemically links FITC to SiO2 NPs, according to Sui et al. (Sui et al., 2014). To label the nanoparticles, we combined by stirring 4 mg of FITC, 2 mL of absolute ethanol and 20 μL APTMS in the dark for 4 hr and then mixed the solution with SiO2 NPs at a SiO2 NP-to-APTMS ratio of 1:0.1.
24 hour mortality testL4-stage nematodes were transferred to 96-well plates with one worm per well. After exposure to SiO2 NPs for 24 hr, worm deaths were counted. Death was defined as when the nematode was immobile and did not respond to mechanical stimuli. E. coli OP50 was added to the nanoparticle suspensions as a food source. Thirty worms were used in each treatment.
Growth, fecundity, and locomotion behaviorNematode growth was assessed by body length and reproductive capacity assessed by brood size. In the growth assay, worms were treated with 0.25-1 mg/mL SiO2 NPs for 24 hr after which, twenty nematodes were randomly selected from each group and anesthetized with 0.1% sodium azide. Nematode length was observed under a microscope, photographed, and the image analyzed with Image-Pro Express. For the fecundity assay, nematodes treated with SiO2 NPs were transferred to NGM plates (one worm per plate). After 12 hr, the worm was transferred to a new plate and the number of eggs counted and this was repeated until the nematode stopped laying eggs. Total fecundity is measured by the total number of eggs and 72 hr-fecundity means the number of eggs produced in the first three days. Twenty worms were examined per treatment.
Head thrash and body bend indicators were used to asses locomotion behavior. Treated nematodes were dispensed onto NGM plates without bacteria and 100 uL K-medium was added. After 30 sec adaptation, the number of head thrashes in 1 min were counted. The movement of the nematode relative to a wavelength on the long axis of the body is defined as one bend of the body. The number of body curvature of nematode within 20 sec was recorded as the body bend index. Twenty nematodes in each group were measured.
Germ cell apoptosis assayGerm cell apoptosis was assessed as previously described (Du et al., 2015). Briefly, nematodes treated with SiO2 NPs were stained with AO and then taken out and placed on NGM plates for 40 min to recover. Nematodes were immobilized by levamisole and observed under an Olympus 1X71 microscope (Olympus, Tokyo, Japan). Normal gland cells were green and apoptotic gonad cells were bright yellow or orange. Thirty nematodes in each group were examined.
Reactive oxygen species (ROS) measurementAfter 24 hr treatment with SiO2 NPs, worms were transferred to 50 μM DCFH-DA solution and incubated in the dark for 30 min. After labeling, a laser-scanning confocal microscope (Leica, TCS SP2, Bensheim, Germany) was used to detect the worms using excitation and emission wavelengths of 488 and 510 nm respectively. The relative fluorescence intensity of intestinal ROS signals was measured using LCS Lite. Twenty worms were detected in each group.
Oxidative stress marker quantificationTreated nematodes were collected in 1.5 mL centrifuge tubes and washed 3 times with MilliQ water. After centrifugation, the supernatant was discarded and the nematodes weighed (mass of nematode = mass of centrifuge tube with nematode - mass of empty centrifugal tube).
GST assayNematodes and precooled protein removal reagent S were ground by Tissuelyser-48 (Shanghai, China), then centrifuged at 10000 g at 4°C for 10 min, and the supernatant retained. The GSH assay kit was used according to manufacturer’s instructions and the absorbance at 412 nm measured with a BioTek fluorescence microplate reader (Winooski, VT, USA).
MDA assayMDA extraction reagent and nematodes were ground by Tissuelyser-48, then centrifuged at 12000 g at 4°C for 5 min, and the supernatant was retained. The MDA assay kit was used according to manufacturer’s instructions and the absorbance at 532 nm was measured with a BioTek fluorescence microplate reader.
Antioxidant co-treatmentTo determine whether the effect of SiO2 NPs on nematodes was related to oxidative stress, we treated nematodes with 10 mM NAC (a common antioxidant) and SiO2 NPs for 24 hr. Specific treatment conditions of each group were: NAC (10 mM), 0.25 mg/mL SiO2 NPs + NAC (10 mM), 0.5 mg/mL SiO2 NPs + NAC (10 mM), and 1 mg/mL SiO2 NPs + NAC (10 mM).
Quantitative real-time polymerase chain reaction (qRT-PCR)RNA was extracted from age-synchronized L3 nematodes exposed to SiO2 NPs for 24 hr using Trizol (Invitrogen, Carlsbad, CA, USA). The High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used for reverse transcription of the total nematode RNA. ABI 7500 FAST Real-Time PCR System (Applied Biosystems) was used to assess mRNA expression. Primers were constructed based on information in the C. elegans (www.wormbase.org) and NCBI databases (www.ncbi.nlm.nih.gov). Specific primer sequences are described in Table 1. Each sample was tested three times to eliminate technical errors, and the independent experiment was repeated three times.
| Gene | Primer sequence (5’-3’) |
|---|---|
| sod-1 | F: TCAGGTCTCCAACGCGATTT R: ACCGGGAGTAAGTCCCTTGA |
| sod-2 | F: GAGGCGGTCTCCAAAGGAAA R: CGCTCTTAATTGCGGTGAGC |
| sod-3 | F: CTCCAAGCACACTCTCCCAG R: TCCCTTTCGAAACAGCCTCG |
| sod-4 | F: GACGCGGTACTTCAGACCAA R: CTGGAGGAAGGGATGCTGTC |
| sod-5 | F: CCGATAAGGTGGTCAGCCTC R: CAAAGACTCCTCGGCCTTGT |
| ctl-1 | F: GTGTCGTTCATGCCAAGGGAG R: TGGATTGCGTCACGAATGAAG |
| ctl-2 | F: TCCCAGATGGGTACCGTCAT R: GGTCCGAAGAGGCAAGTTGA |
| ctl-3 | F: ATGCCAATGCTTCCCCACAT R: GCAGGTGGGGTTCCTGATTT |
| hus-1 | F: CGGCGGACACTGTTATCTGA R: AATTGACGGCCTGGAGTACG |
| cep-1 | F: AGAATACCCGATTCGCAGGAC R: TCGCCATTGCCCAGTATTCC |
| ced-13 | F: ACACTTTCTCCCGCTGTTGT R: TCAGAGTCAAACTCGTCGCA |
| ced-9 | F: AGTGATGCTCAGGACTTGCC R: AGCCTTGGCTCTTCCCAATC |
| ced-4 | F: GCTGATGCTAAAAAGCGAAGA R: CGTTGCTGGATTTCCACTGC |
| ced-3 | F: GGAGCTTGCTAGAGAGGAACA R: TCAGCAAGTCCTTCGTGTCC |
| mtl-1 | F: AGTGCGGAGACAAATGTGAATGC R: AGCAGTTCCCTGGTGTTGATGG |
| mtl-2 | F: TTGTTCCTGCAACACCGGAA R: GTTGGCACACTTGCATCCTC |
| actin | F: GCTGGACGTGATCTTACTGATTACC R: GTAGCAGAGCTTCTCCTTGATGTC |
All data from at least three independent experiments were expressed as the mean ± standard deviation (SD). Statistical analyses were performed using IBM SPSS Statistics 22. One way analysis of variance (ANOVA) was used for significance analysis, and a Tukey post-hoc test was used for multiple comparisons. Significant differences were defined as P < 0.05.
Morphology and size of SiO2 NPs was examined by TEM (Fig. 1a). The size of SiO2 NPs, as measured by Image J, was mostly within the range of 25-35 nm (Fig. 1b). The average size was 30.4 ± 5.7 nm, which is consistent with the data provided by the supplier (30 nm). The mean hydrodynamic size and the zeta potential of SiO2 NPs in K-medium were measured by Malvern Zetasizer Nano S90 (Table 2). Figure 1c shows that the hydrodynamic size of 0.25 and 0.5 mg/mL SiO2 NPs is mostly stabilized at about 700 nm within 24 hr, while that of 1 mg/mL SiO2 NPs is stable at around 850 nm.
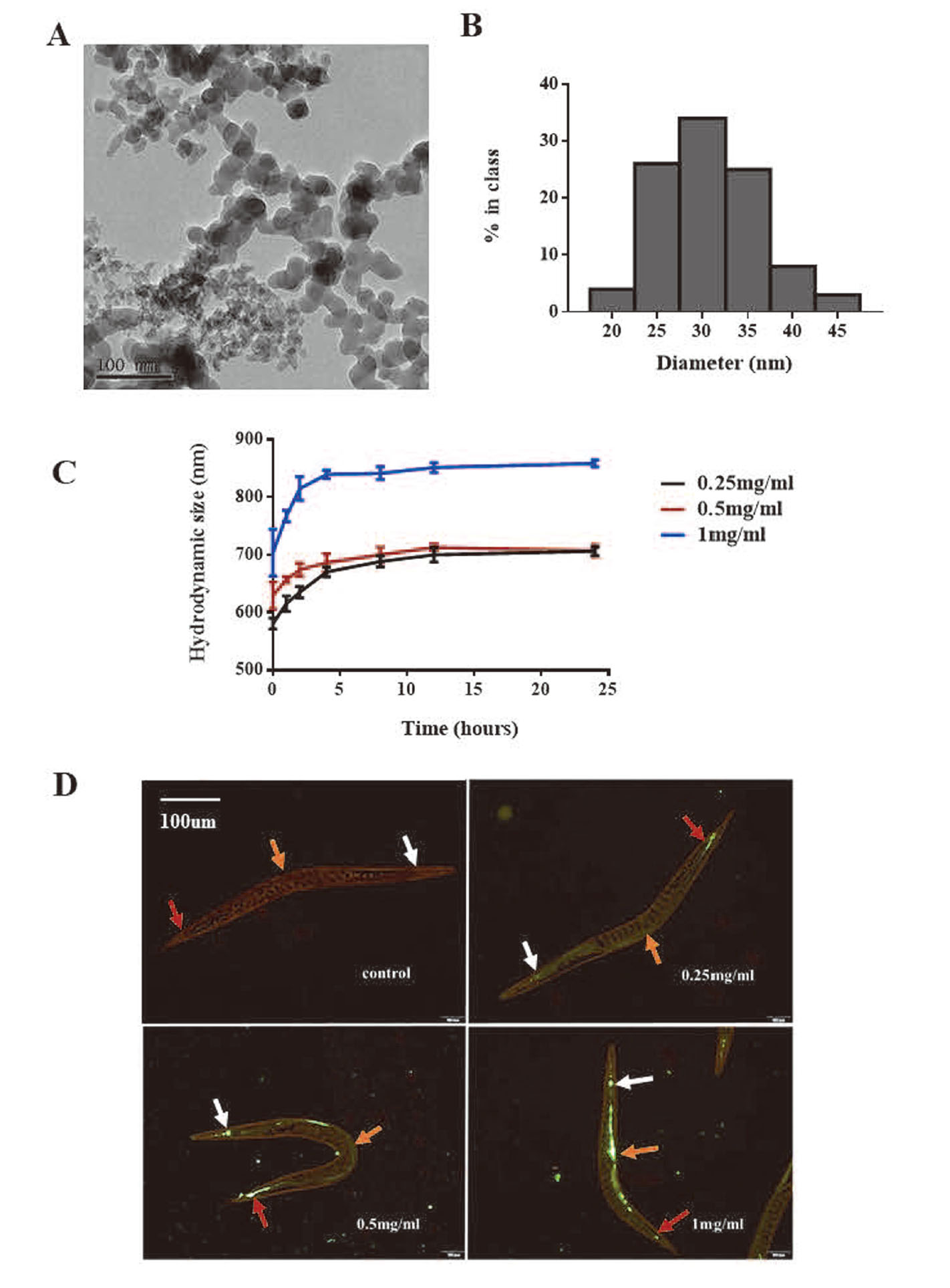
SiO2 NPs characterization and the nanoparticles in vivo. (A): TEM image of SiO2 NPs. (B): Particle size distribution of SiO2 NPs (calculated with 200 nanoparticles by ImageJ) (C): The hydrodynamic size of SiO2 NPs in K-medium at 0, 1, 2, 4, 8, 12, and 24 hr, respectively (each sample was tested three times at each time point). (D): Distribution of FITC-labeled SiO2 NPs in nematodes. (10×) white arrow indicates worm pharynx, orange arrow indicates worm intestine, red arrow indicates worm tail.
| Concentration (mg/mL) | Hydrodynamic size in K-medium (nm)* | Zeta potential (mV)* | ||||
|---|---|---|---|---|---|---|
| 0.25 (SiO2 NPs) | 580.5 | ± | 9.7 | -27.4 | ± | 6.3 |
| 0.25 (FITC-SiO2 NPs) | 596.0 | ± | 13.5 | -24.1 | ± | 3.6 |
| 0.5 (SiO2 NPs) | 629.2 | ± | 23.5 | -22.2 | ± | 7.5 |
| 0.5 (FITC-SiO2 NPs) | 667.4 | ± | 32.1 | -22.9 | ± | 4.1 |
| 1 (SiO2 NPs) | 703.5 | ± | 40.8 | -18.6 | ± | 3.6 |
| 1 (FITC-SiO2 NPs) | 737.8 | ± | 55.3 | -16.9 | ± | 3.6 |
* Hydrodynamic size in K-medium and zeta potential were determined through Malvern Zetasizer Nano S90, and each sample was tested three times.
To check whether SiO2 NPs were ingested by the animal, we used fluorescence microscopy to examine the worms after they were treated with the FITC- SiO2 NPs for 24 hr. The mean hydrodynamic size and the zeta potential of FITC- SiO2 NPs in K-medium are shown in Table 2, and are similar those of SiO2 NPs. Figure 1d shows green fluorescence in the pharynx, intestines and tail of the nematodes, and fluorescence intensity increased when the FITC-labeled SiO2 NP concentration increased.
SiO2 NPs effects on nematode survival, development, fecundity, and locomotionTo understand the effects of SiO2 NPs on nematodes, we assessed some common health parameters after 24 hr nanoparticle exposure. We found that exposure to 0.25-1 mg/mL SiO2 NPs did not affect nematode survival (Fig. 2a). Worm body lengths were only significantly reduced (P < 0.05) at 1 mg/mL SiO2 NPs (Fig. 2b). Obvious reductions were seen in 72 hr and total fecundity after SiO2 NP treatment at all concentrations, which were statistically significant (Figs. 2c and 2d). These results suggest that fecundity is more sensitive to SiO2 NPs toxicity than survival and body length. SiO2 NPs were shown to have different degrees of adverse effects on nematode body bends and head thrashes (Figs. 2e and 2f).
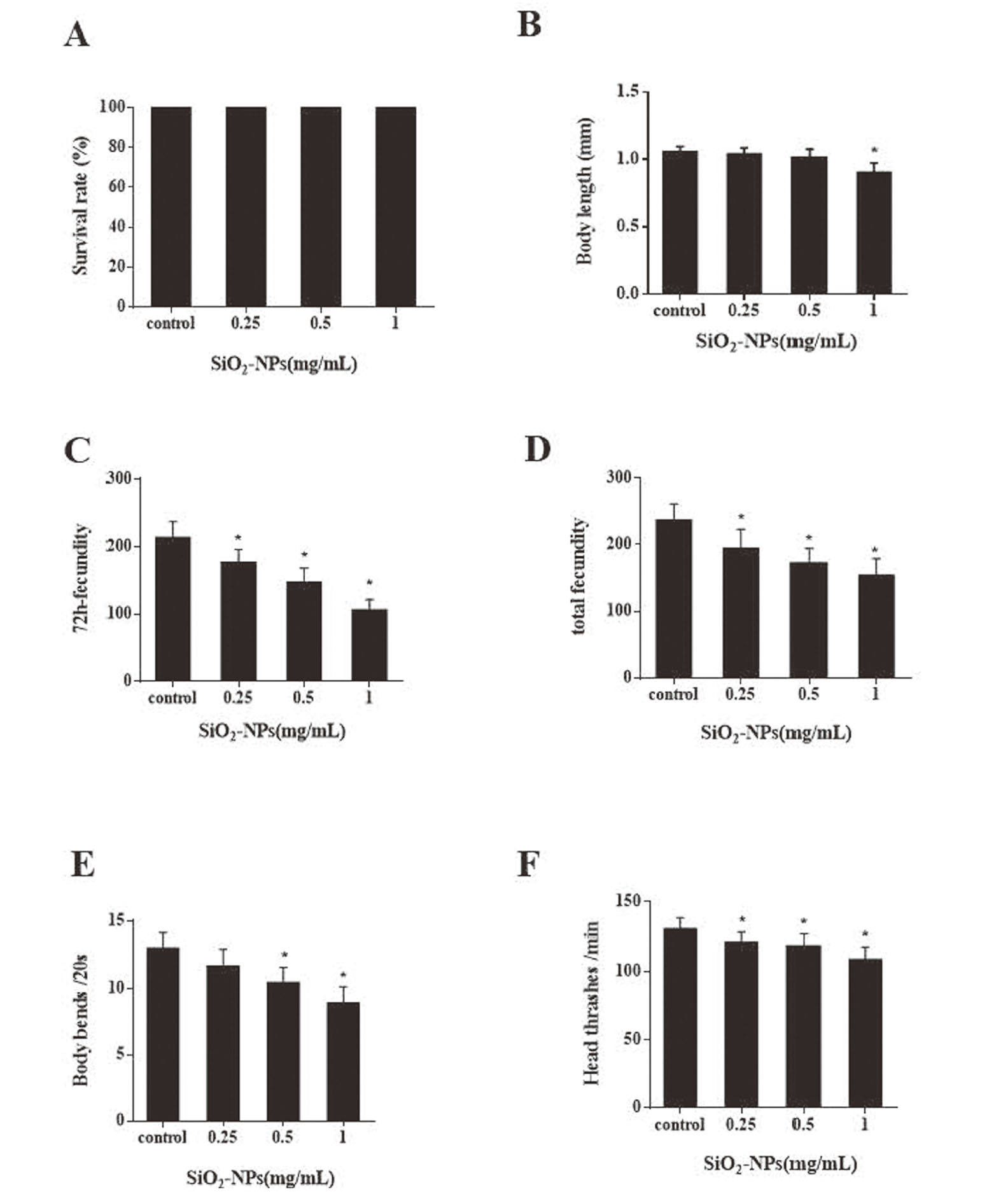
SiO2 NPs effects on nematode survival, development, fecundity, and locomotion. C. elegans were exposed to 0, 0.25, 0.5 and 1 mg /mL SiO2 NPs for 24 hr. (A) Effects of SiO2 NPs on survival of nematodes. (B) Effects of SiO2 NPs on development of nematodes. The development was assessed by body length. (C&D) Effects of SiO2 NPs on 72 hr-fecundity and total fecundity of nematodes. (E&F) Effects of SiO2 NPs on locomotion of nematodes. The locomotion was assessed by body bends and head thrashes. Each experiment was independently repeated three times, bars represent mean ± SD. * P < 0.05.
Previous studies have shown that SiO2 NPs reduce the activity and quantity of sperm in mice and are associated with apoptosis of spermatogenic cells (Ren et al., 2016, 2019). In our experiments, SiO2 NPs exposure significantly reduced the reproductive ability of nematodes, so we speculated that nanoparticles may enhance the apoptosis of germ cells. Apoptotic germ cells appeared yellow-green after AO staining (Fig. 3a). In the control and 0.25, 0.5 and 1 mg/mL SiO2 NP-treated nematodes, an average of 1.4, 2.5, 4.1, and 6.3 apoptotic cells respectively were observed (Fig. 3b). Apoptotic cell numbers in the treated groups were significantly higher (P < 0.05) than the controls, and in the 1 mg/mL group was, on average, nearly 4 times higher than the control.

SiO2 NPs enhanced germ cell apoptosis. (A) Representative images of apoptotic cells following AO staining in worms after exposure to SiO2 NPs for 24 hr. Apoptotic cells appear as solid green dots indicated by red arrows. (20×) (B) Average number of apoptotic cells per gonad arm after exposed to SiO2 NPs for 24 hr. Each experiment was independently repeated three times, bars represent mean ± SD. * P < 0.05.
At present, there is no unified conclusion about the mechanism of SiO2 NPs toxicity. Previous studies have shown that oxidative stress is one mechanism (Gong et al., 2012; Rajiv et al., 2016). To investigate whether SiO2 NPs induce oxidative stress after 24 hr treatment, ROS, MDA and GSH indexes were calculated. As shown in Figs. 4a and 4b, both 0.5 and 1 mg/mL treatments significantly increased ROS production compared to the controls (P < 0.05). MDA increased, and GSH decreased, in a dose dependent manner (Figs. 4c and 4d). These results indicate that SiO2 NPs influence oxidative stress levels in nematodes.
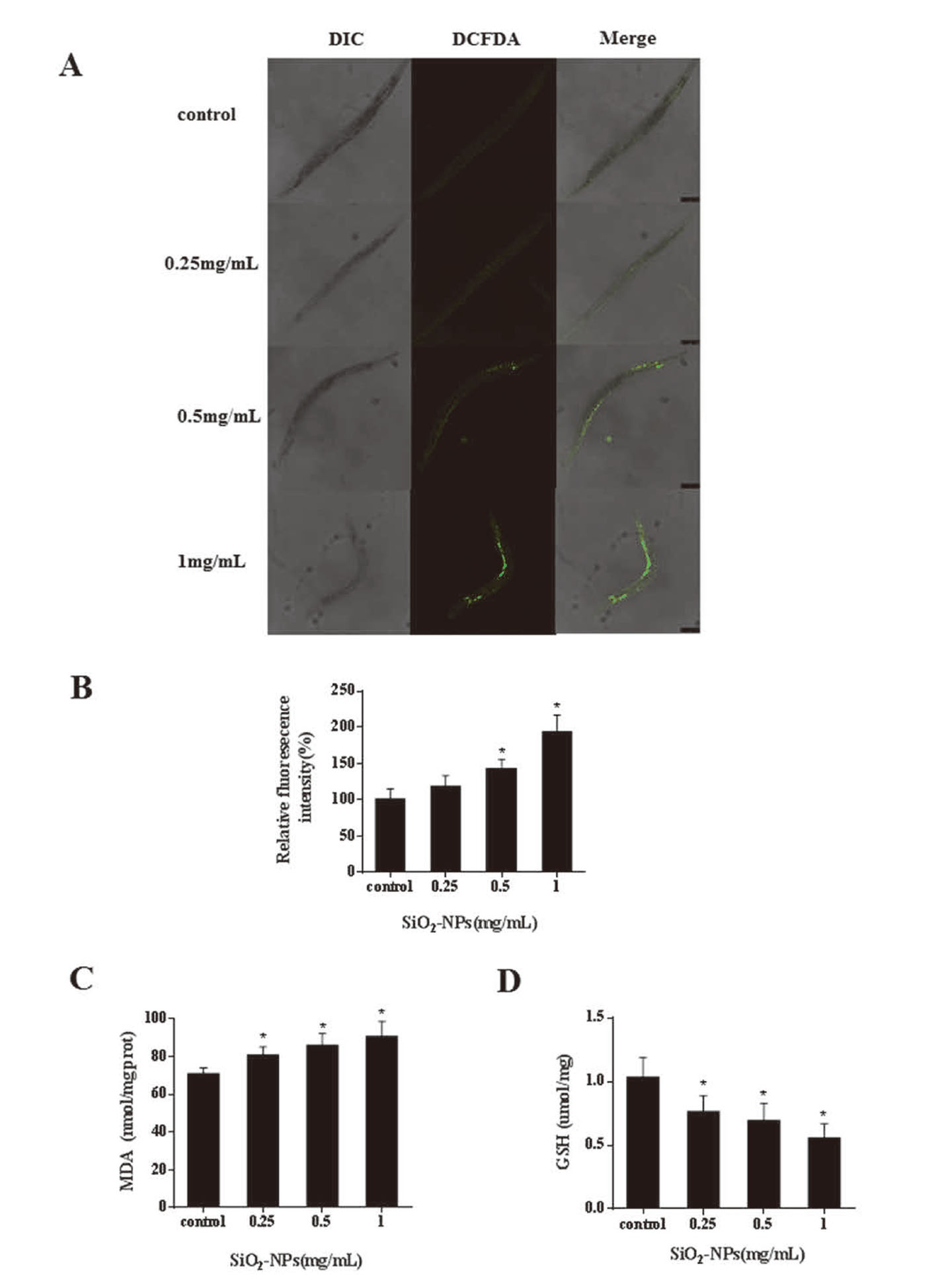
SiO2 NPs induced an increase in ROS and affected GST and MDA levels. (A) Effects of SiO2 NPs on reactive oxygen species (ROS) in Caenorhabditis elegans were detected by H2DCFDA fluorescence probe. (10×) (B) Comparison of ROS production in nematodes exposed to different concentrations of SiO2 NPs. (C) Effects of SiO2 NPs on malondialdehyde (MDA) of nematodes. (D) Effects of SiO2 NPs on reduced glutathione (GSH) of nematodes. Each experiment was independently repeated three times, bars represent mean ± SD. * P < 0.05.
We hypothesized that germ cell apoptosis induced by SiO2 NPs was regulated by oxidative stress. Nematodes were exposed to SiO2 NPs and NAC (10 mM), an antioxidant, and germ cell apoptosis was measured after 24 hr. NAC was shown to eliminate the effects of SiO2 NPs on nematode germ cells at all concentrations examined (Figs. 5a and 5b). This further supports the theory that SiO2 NPs enhance germ cell apoptosis by inducing ROS. We also examined the reproductive capacity of worms co-exposed to SiO2 NPs and NAC and the results showed that both 72 hr and total fecundity was restored (Figs. 5c and 5d).
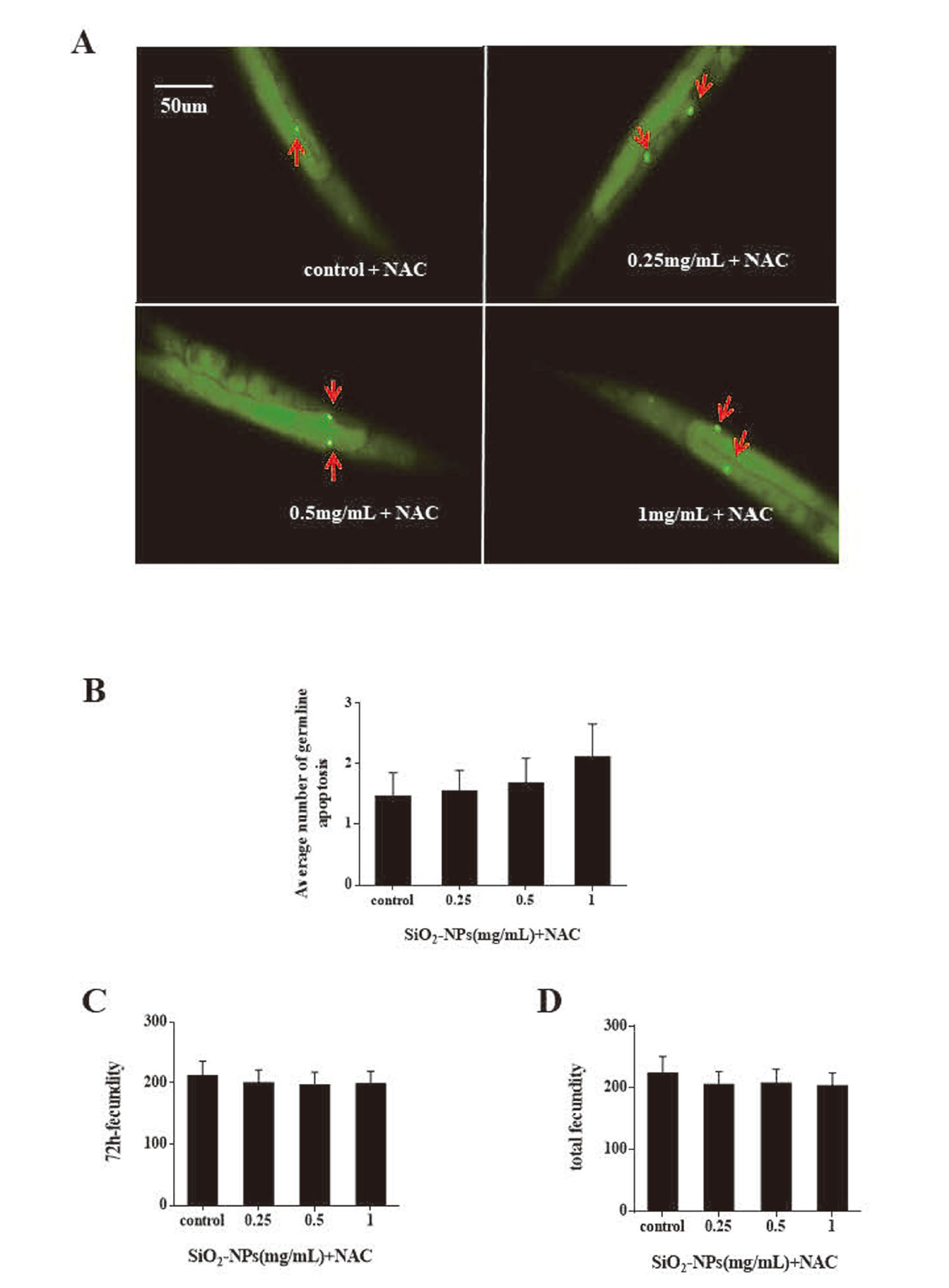
NAC ameliorated SiO2 NPs reproductive toxicity. (A) Representative images of apoptotic cells following AO staining in worms after co-exposure to SiO2 NPs and NAC (10 mM) for 24 hr. Apoptotic cells appear as solid green dots indicated by red arrows. (20×) (B) Average number of apoptotic cells per gonad arm after co-exposed to SiO2 NPs and NAC (10 mM) for 24 hr. (C&D) Effects of SiO2 NPs and NAC (10 mM) on 72 hr-fecundity and total fecundity of nematodes. Each experiment was independently repeated three times, bars represent mean ± SD. * P < 0.05.
To uncover the molecular mechanisms of SiO2 NP toxicity we examined the expression of genes related to oxidative stress (sod-1,sod-2,sod-3,sod-4,sod-5,ctl-1, ctl-2, ctl-3), apoptosis (hus-1, cep-1, ced-13, ced-9, ced-4 and ced-3) and metal detoxification (mtl-1 and mtl-2). The results showed that, in comparison to the control group, the expression of ced-13 in nematodes treated with 0.25, 0.5 and 1 mg/mL SiO2 NP was significantly upregulated (p < 0.05), while that of sod-4 and ctl-1 was significantly downregulated (p < 0.05). mRNA levels of sod-1, sod-2, sod-5, hus-1, cep-1, ced-4, and ced-3 were significantly increased (p < 0.05) and levels of mtl-1, mtl-2 and ctl-2 significantly decreased (p < 0.05) after 24 hr exposure to 0.5 and 1 mg/mL SiO2 NPs. The mRNA levels of sod-3 and ced-9 (homolog of the mammalian cell-death inhibitor Bcl-2) increased at 0.5 mg/mL but decreased at 1 mg/mL. The expression levels of ctl-3 were unaffected by SiO2 NP treatment. Among the tested genes, mtl-1 and mtl-2 expression levels changed the most and decreased by 90% and 70%, respectively, in worms treated with 1 mg/mL SiO2 NPs (Fig. 6).
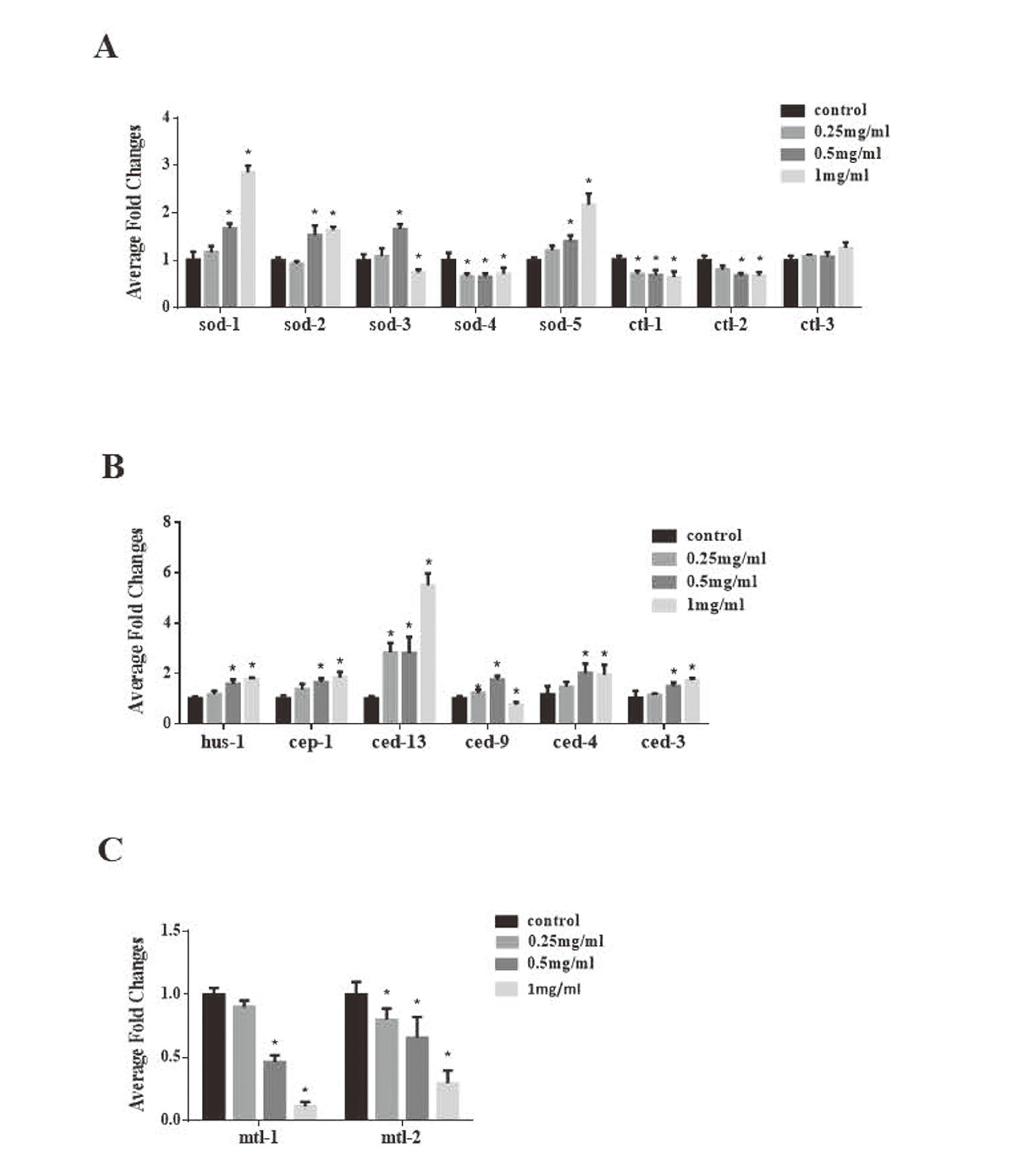
SiO2 NPs effects on nematode gene expression. (A) Gene expression fold changes of genes that are related to oxidative stress. (B) Gene expression fold changes of genes that are related to apoptosis. (C) Gene expression fold changes of genes that are related to metal detoxification. Determined by qRT-PCR, actin was taken as an internal control. Each experiment was independently repeated three times, * P < 0.05.
Silica nanoparticles are widely used in scientific research, production and daily life, and can enter the body through a variety of different routes, but we currently know very little about their oral toxicity. In this study, we exposed nematodes to 30 nm SiO2 NPs and studied the effects on survival, development, reproduction, and movement, in order to make a preliminary evaluation of the oral safety of nanoparticles. We also examined the reproductive toxicity of SiO2 NPs and explored their molecular mechanisms of action.
Surface chemical properties can affect the interaction between nanoparticles, and while size is the most important factor affecting their toxicity this does not, however, simply mean that the smaller the particle size, the greater the toxicity (Hubbs et al., 2011). Chithrani and Chan noted that the small size of nanoparticles does not result in better absorption and increased toxicity (Chithrani et al., 2006). In this study, nanoparticle size was confirmed by TEM, and SiO2 NPs were observed in the in the pharynx, intestines, and tail of nematodes. A previous study showed that rhodamine-labeled SiO2 NPs could also be seen in the spermathecae, in addition to the throat and intestines of the worms (Scharf et al., 2013). The discrepancies between findings may be due to nanoparticle sizes or differences in the fluorescent labels.
The nematode’s reproductive ability is more sensitive to SiO2 NPs toxicity than survival, development, and behavioral ability, which is consistent with previous studies. Eom and Choi observed significantly reduced reproductive capacity in nematodes exposed to SiO2 NPs (11 nm) for 24 hr (Eom and Choi, 2019). Pluskota et al. showed that nematode exposure to SiO2 NPs increased the worm bag phenotype, resulting in decreased reproductive ability (Pluskota et al., 2009). The worm bag phenotype was not present in our study, which may be due to nanoparticle size and surface modification.
While the underlying molecular mechanisms of reproductive toxicity of nanoparticles are not fully understood, possible mechanisms include oxidative stress, apoptosis, inflammation, and genotoxicity (Wang et al., 2018). Apoptosis is a highly-conserved process which plays an important role in cell development and homeostasis (Hengartner, 1997). Generally, over 300 germ cells will undergo apoptosis in adult worms and at any time during the first 3-4 days of adulthood 2-3 apoptotic gonad cells can be observed (Gumienny et al., 1999). After exposure to SiO2 NPs for 24 hr, the number of apoptotic gonad cells significantly increased. The expression levels of apoptosis-related genes (cep-1, ced-13, ced-4, ced-3) were also increased. Gartner et al. found that germ cells undergo apoptosis induced by DNA damage, rather than somatic cells (Gartner et al., 2000). The DNA damage checkpoint gene hus-1, which is essential for cell cycle blocking and apoptosis induction by DNA damage, was significantly (p < 0.05) upregulated after SiO2 NP exposure (Fig. 6). These results suggest that DNA damage may be involved in SiO2 NP-induced apoptosis.
Oxidative stress is one of the most important causes of male infertility and ROS can damage both sperm function and DNA integrity (Hussein et al., 2016). Previous studies have shown that oxidative stress is closely related to nanoparticle toxicity (Patel et al., 2018). ROS, GSH and MDA levels were measured to assess the state of oxidative stress. ROS are a class of oxygen-containing compounds that are produced during aerobic metabolism and include peroxide (H2O2), superoxide anion (O2-), and hydroxyl radicals (OH) (Abdal Dayem et al., 2017). MDA is the product of lipid peroxidation and GSH is a major antioxidant in cells which protects the body from oxidative stress-related toxicity and damage caused by scavenging free radicals and hydrogen peroxide (Tsikas, 2017; Owen and Butterfield, 2010). Our results showed that SiO2 NPs could increase the oxidative stress levels in nematodes and that the addition of NAC, an antioxidant, could restore the damage caused by SiO2 NPs. Based on this, we speculate that SiO2 NPs can enhance the apoptosis of gonad cells by inducing oxidative stress which will damage reproductive ability.
To further explore the mechanism of toxicity of SiO2 NPs, we examined the expression levels of oxidative stress-related genes. The five nematode sod genes (sod-1, sod-2, sod-3, sod-4, sod-5) are homologs of mammalian cytoplasmic, mitochondrial, and extracellular superoxide dismutase (SOD1, SOD2, SOD3), and the three nematode ctl genes (ctl-1, ctl-2, ctl-3) are homologs of mammalian cytoplasmic and peroxisomal catalase (CAT) (Sharma et al., 2018). The sod genes are involved in superoxide dismutase and protein homodimerization activity, and the ctl genes are related to catalase activity (Zelko et al., 2002; Song et al., 2014). In this study, sod-1, sod-2 and sod-5 were up-regulated, while sod-4, ctl-1 and ctl-2 were down-regulated, further confirming that SiO2 NPs can induce oxidative stress. Of the tested genes, the expression levels of mtl-1 and mtl-2, which encode metallothioneins, changed the most. Metallothionein is a protein with special physiological functions and plays a unique role in reducing the toxicity of heavy metals such as cadmium, nickel, and lead, as well as scavenging excessive free radicals, anti-aging, and tumor (Bhandari et al., 2017). Previous studies have shown that metallothionein can provide a stable redox environment and protect cells from oxidative stress by resisting the stress response of various organelles (Rios et al., 2018). Polak et al. have shown that mtl-1 and mtl-2 play an important role in ameliorating the cytotoxicity of ZnO NPs (Polak et al., 2014). We conclude from this that the toxicity of SiO2 NPs may depend on metallothionein activity.
In conclusion, the toxic effects of SiO2 NP on Caenorhabditis elegans were explored in this study, which provides a reference for evaluating the oral toxicity of SiO2 NP. We detected gonad cell apoptosis and oxidative stress injury, clarified the relationship between them, and provided clues for the mechanism of SiO2 NP toxicity. In addition to this, the correlation between the toxicity of SiO2 NP and metallothionein was preliminarily revealed by the detection of related genes. In future studies, according to the screening of oxidative stress-related genes, we plan to use nematode mutants to help further uncover the mechanism of SiO2 NP toxicity, and explore the interaction between metallothionein and SiO2 NP induced oxidative stress.
This study was funded by Shanghai Jiao Tong University Medicine-Engineering Research Fund (Grant No.YG2019QNA71) and Startup Fund for Youngman Research at Shanghai Jiao Tong University (SFYR at SJTU 17X100040015)
Conflict of interestThe authors declare that there is no conflict of interest.